Liquefied petroleum gas (LPG) is a commonly used fuel in many civil, commercial, and industrial applications. So what is LPG, what are its composition and applications? Let's explore LPG in detail in the following article!
What is LPG?
LPG (Liquid Petroleum Gas) is also known as liquefied petroleum gas. Its composition includes a mixture of hydrocarbon gases, mainly propane (C3H8) and butane (C4H10). This mixture of propane and butane is compressed into liquid form for storage at a certain pressure.
At normal temperatures, liquefied natural gas (LPG) vaporizes and is used as a gas. In daily life, LPG is often simply called gas. LPG is colorless and odorless, but in practice, to detect gas leaks, LPG is mixed with a substance called Ethanethiol – this substance does not affect the combustion quality of LPG.

Basic characteristics of liquefied petroleum gas (LPG)
Boiling point: This is the temperature at which a liquid boils under normal ambient pressure (760 mmHg). Since LPG is used in vapor form, the amount of gas supplied depends heavily on the volatility of the liquid gas. LPG with a lower boiling point is easier to use in areas with low temperatures. The boiling point of propane is -42 °C. The boiling point of butane is 0 °C. In Vietnam, the appropriate ratio between propane and butane is 70% and 30%.
LPG saturation pressure: The amount of LPG stored in cylinders is in a saturated state, with liquid gas at the bottom and gas vapor at the top. According to safety regulations, gas cylinders are only allowed to contain 80% of their capacity; the remaining 20% is to allow for the expansion of the liquid gas. Propane has a much higher saturated vapor pressure than butane. The saturated vapor pressure depends on the ambient temperature, not the amount of gas contained within. If the liquid gas temperature increases, the liquid gas will immediately boil and vaporize to restore the saturated pressure. At normal temperatures, the gas has a pressure of 1.7 kg/cm² to 6.8 kg/cm².
Thermal energy: When 1 kg of LPG is completely burned, it provides approximately 11,000 kcal of heat energy. This calorific value is higher than other common fuels such as gasoline, oil, coal…
Density: The density of liquid LPG is 0.55~0.57 kg/liter; LPG vapor density: LPG vapor is almost twice as heavy as air, so when released, the gas lies close to the ground and concentrates in low places such as ditches and manholes…
Expansion ratio: The expansion ratio of liquefied natural gas is very large: One unit volume of liquid LPG creates 250 units volume of LPG vapor. This explains why gas is stored in liquid form. Flame temperature: The flame temperature of propane burning in air is 1930 °C. The flame temperature of butane burning in air is 1900 °C.
Other basic properties: LPG is a clean fuel, when burned it produces a fairly high temperature (about 1900-1950°C), very little soot and does not produce dirt or toxic CO gas. 1.0 kg of LPG, when burned, produces 1200 kcal of heat, equivalent to the heat generated by burning 3-4 kg of coal, 2 liters of kerosene, 1.5 liters of gasoline, or 7.9 kg of firewood.
Applications of LPG
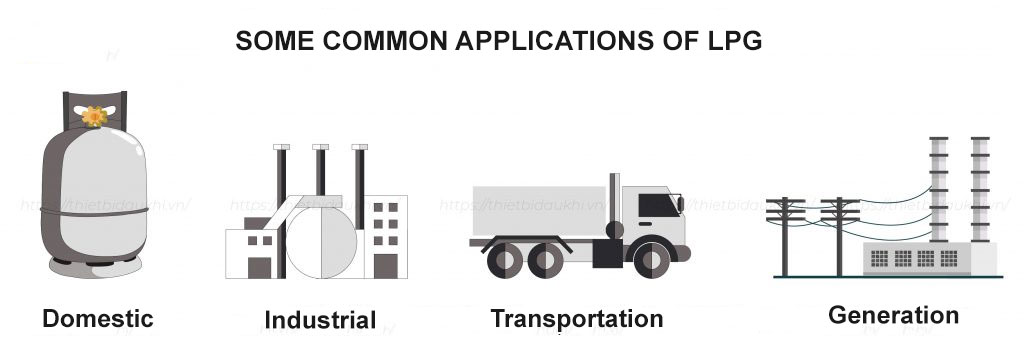
LPG is widely used and can be divided into the following main areas:
Domestic and Commercial: Used as a fuel for daily living activities such as cooking and heating in households, restaurants, hospitals, and schools.
Agricultural and Industrial: LPG is used as a fuel in the industrial, metallurgical, furnace, and electroplating sectors. In agriculture, liquefied petroleum gas is used for drying agricultural products.
Transportation: Fuel for transportation vehicles: spacecraft, cars, buses, etc.
Fuel for Power Generation: Used in the production of ethylene, propylene, and butadiene for the plastics industry, and MTBE, an octane booster that replaces lead in gasoline.